Choosing the Right Phosphorus Method
This article originally appeared in the AGVISE Laboratories Spring 2023 Newsletter under President’s Corner
The phosphorus soil test debate never ends. Should I use the Olsen test, or maybe Bray-1 would be better? What about the Mehlich-3 method, and should that extract be analyzed on an ICP or with a colorimetric method? Perhaps, Bray-2 or the Haney extractable P is something to consider? This whole phosphorus test dilemma can be quite confusing; however, the answer is quite simple. Use the soil phosphorus test that is calibrated for your region!
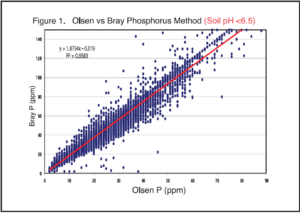
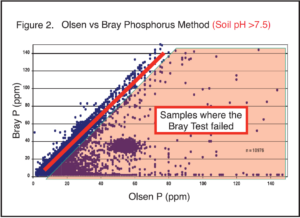
In the upper Midwest, the Olsen test is the most reliable method to determine phosphorus availability and has the most correlation and calibration data with field trials. Many hours have been spent by university researchers putting out field trials to determine phosphorus fertilizer rates for various crops. The researchers have evaluated various phosphorus methods, and the two most common methods are the Bray-1 and Olsen extractants. The Bray-1 method is the older method, developed in Illinois. It works well on soils with pH below 7.3. Once the soil pH is above 7.3, the extractant may fail. If the test fails, it will produce a result near zero.
The Olsen method is required on calcareous soils (pH > 7.3), but it also works well on acidic soils. There is a common misconception that the Olsen method is only suitable on calcareous soils. In fact, the Olsen method is widely used across the world because of its versatility on acidic and calcareous soils. It is a perfect fit for our region because it works so well across a wide soil pH range and on diverse soil types. In the AGVISE Newsletter Spring 2017 issue, retired AGVISE President Robert Deutsch compiled soil test data for the Bray-1 and Olsen methods with over 25,000 soil samples. The graphs highlight how robust the Olsen phosphorus method is, working on acidic and calcareous soils alike.
The Mehlich-3 method has gained popularity in the southeast United States and the central Midwest. In these regions, the soils are more weathered and often do not have problems with high calcium carbonate content. At the University of Minnesota, Dr. Dan Kaiser has worked on Mehlich-3 method correlation on Minnesota soils for quite a few years. For some soils, the Mehlich-3 method performed as expected, while some others had Mehlich-3 results 8 to 10 times higher than expected. For these reasons, the Mehlich-3 method has not been approved for use in the upper Midwest or northern Great Plains.
As of this time the only phosphorus soil tests recommended for soils in the upper Midwest are the Olsen and Bray-1 extracts. If someone mentions using any other phosphorus soil test, it has not been tested or correlated to the soils in this region.