Soil Science Review: Soil pH, Acidity, and Alkalinity
Soil pH is a basic soil property that affects many biological and chemical processes in soil. Simply knowing if a soil is acidic or alkaline can tell us a lot about how it behaves and how we can manage it. This is why soil pH is often called the master variable of biological and chemical reactions.
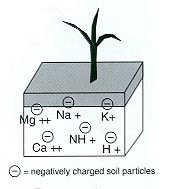
Soil pH is the activity of hydrogen ions (H+) in the soil solution, expressed on a logarithmic scale. A neutral soil has pH 7.0 and contains equal parts hydrogen (H+) and hydroxide (OH–) ions. An acidic soil has more H+ ions. An alkaline soil has more OH– ions. The relative acidity or alkalinity is shown in Table 1. In the laboratory, soil pH is analyzed using the 1:1 soil:water ratio routine method. Other soil pH methods include CaCl2, KCl, and saturated paste.
| Table 1. Relative soil acidity or alkalinity range from soil pH. | |
| pH (1:1) | Relative Acidity or Alkalinity |
| ≤ 4.4 | Extremely acidic |
| 4.5-4.9 | Very strongly acidic |
| 5.0-5.4 | Strongly acidic |
| 5.5-5.9 | Moderately acidic |
| 6.0-6.4 | Slightly acidic |
| 6.5-7.5 | Neutral |
| 7.6-7.9 | Slightly alkaline |
| 8.0-8.4 | Moderately alkaline |
| 8.5-8.9 | Strongly alkaline |
| ≥ 9.0 | Very strongly alkaline |
The optimal pH range for most plant species is near neutral or slightly acidic. In the optimal pH range, most plant nutrients are at or near their highest solubility in the soil solution. If soil pH is too low or too high, the availability of plant nutrients decreases; therefore, soil pH may be corrected with soil amendments or other strategies to mitigate reduced nutrient availability.
To demonstrate the importance of soil pH, let’s look at soil pH and aluminum. Aluminum is a natural component of soil clay particles, and it is insoluble above pH 5.5. In strongly acidic soils (pH < 5.5), aluminum solubility increases, so aluminum begins to dissolve and enter the soil solution. Soluble aluminum is very toxic to plant root growth and development, and it may cause reduced plant production or plant death. Soluble aluminum also binds with phosphate in the soil solution to create insoluble aluminum phosphate compounds, which then reduce soil phosphorus availability and plant uptake.
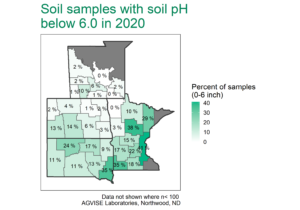
Soil acidity and aluminum toxicity is often the primary limitation of crop production in tropical and subtropical regions. Acidic soils are frequently amended with lime (calcium carbonate) to increase soil pH, improve nutrient availability, and increase crop production. On the glaciated plains of North America, soil acidity is not a common phenomenon. However, some localized areas of long-term no-till crop production on coarse-textured soils has produced more soils with very low pH (<5.0) and new aluminum toxicity problems.
Soil alkalinity similarly reduces the availability of plant nutrients in soil. In moderately alkaline soils (pH > 8.0), phosphorus binds with calcium to create insoluble calcium phosphate compounds, which then reduce soil phosphorus availability and plant uptake. Similarly, the micronutrients iron and zinc are less soluble. To improve nutrient availability in alkaline soils, farmers apply fertilizer in narrow bands. These bands decrease the volume of soil with which the fertilizer can react, thus keeping more nutrients available in the soil solution. It is generally uneconomical to lower alkaline soil pH to the optimal pH range in crop production.
Soil pH goes beyond inorganic soil chemistry. It also controls the biological activity of soil microorganisms that help create soil structure, cycle organic nutrients, and fix nitrogen in the nodules on legume roots. Soil pH also controls the degradation of many pesticides in soil. If there is something going on in soil, it probably starts with pH.