Active carbon (POXC): What does it measure?
Carbon is the currency of nature: the backbone of soil organic matter and the energy source for soil microorganisms. Therefore, much interest in soil health focuses on increasing carbon storage in soil. When you reduce tillage or increase crop rotation diversity, you expect soil organic matter to increase. However, soil organic matter often changes slowly for several years. In fact, less than 1% of plant biomass carbon returned to soil eventually becomes stable humus organic carbon.
Active carbon, also known as permanganate-oxidizable carbon (POXC), is a sensitive tool for measuring soil carbon change. This portion of soil organic matter is actively involved in nutrient cycling and changes more quickly when cropping systems are changed (e.g. reduced tillage, diversified crop rotation, cover crop inclusion). Active carbon is a quick, repeatable soil test that measures the easily oxidizable, biologically active carbon fraction.
Why is active carbon important?
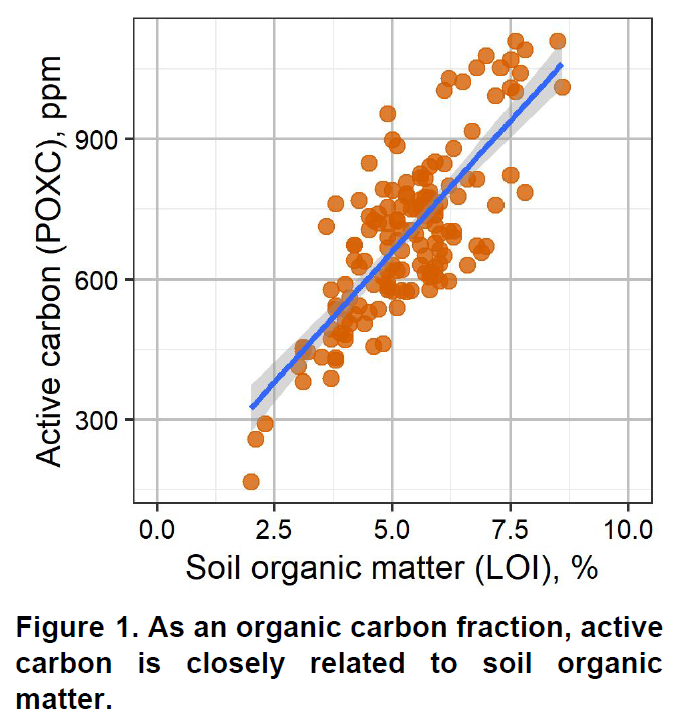
Active carbon typically comprises about 1 to 4% of total organic carbon in soil. It represents the microbially available carbon energy sources, that is microorganism food. As one component of the total organic carbon pool, active carbon has a strong relationship with overall soil organic matter (r = 0.80, Fig. 1), but it responds more quickly to changes in crop and soil management. It helps explain why two soils with 3% soil organic matter, for example, may behave differently regarding biological activity or nutrient cycling.
Active carbon should be utilized as a tracking tool to measure improvement in soil quality. In a 17-year tillage experiment in Mandan, ND, total organic carbon did not change much after conversion to no-till; however, active carbon increased significantly from 470 to 600 ppm (Weil et al., 2003). Recall that soil organic matter includes all forms of organic carbon (i.e. microbial biomass, recently decomposed plant material, stable humus) and requires large carbon inputs to change that total measurement. In contrast, the active carbon fraction increases much more quickly and detects improvements in soil quality sooner. This is why active carbon is considered a “leading” soil health indicator.
What do the numbers mean?
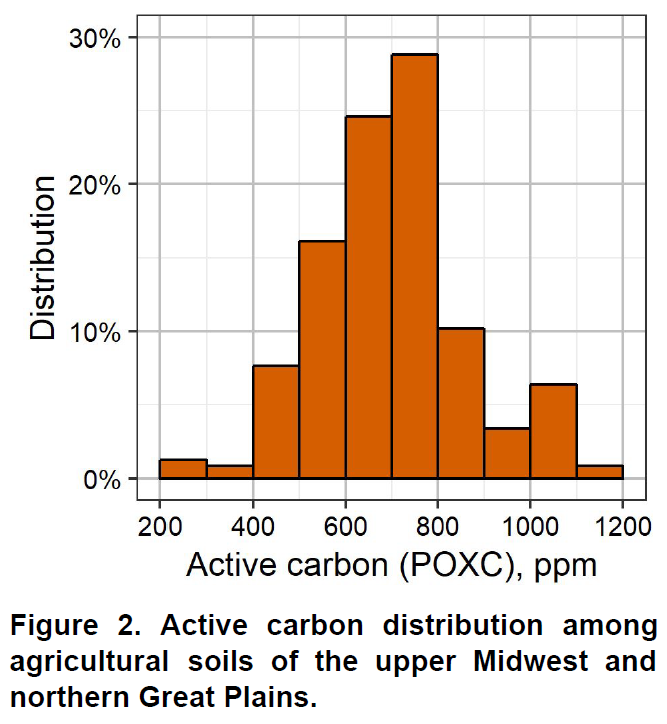
In agricultural soils of the upper Midwest and northern Great Plains, active carbon commonly ranges from less than 300 ppm to 1000 ppm (Fig. 2). As with soil organic matter, soil texture is a major factor controlling active carbon. Coarse-textured soils generally contain less active carbon than medium- or fine-textured soils. Native prairie soils may contain active carbon as high as 1500 ppm.
While these are the common active carbon values you may expect, each field and its history is different; therefore, making comparisons between fields is not advised. There are no index ranges for active carbon because different climatic regions and soil types cannot be judged by the same standard. Active carbon should be utilized as a tracking tool in improving the soil quality of that field or zone, rather than making broadscale comparisons.
Cropping systems that include reduced tillage or no-till, diversified crop rotations, and cover crops will help increase active carbon. In addition, any system with greater organic matter inputs (e.g., plant biomass, manure) helps build active carbon and soil organic matter. Under perennial grass, active carbon can easily exceed 1000 ppm.
With sensitive soil health tools like active carbon, you can more easily quantify positive changes achieved through better soil management.
How to soil sample
Active carbon can be added to any standard soil fertility analysis, simply requested as an analysis add-on. Collect the soil sample with standard soil probe. The standard soil sampling depth of 0-6 inches is most common. In reduced tillage and no-till systems, stratification may warrant 0-2 or 0-4 inch soil sampling depths. Like any soil health test, it is a tracking tool measuring soil quality improvement, so make sure you are using GPS-marked soil sampling points.
Frequently asked questions (FAQ)
Q: Does higher active carbon (POXC) indicate better crop yield?
A: Not necessarily. Crop yield is comprised of numerous determinants: genetics, climate, soil type, soil fertility, crop pests, etc. Active carbon measures the carbon sources available to microorganisms as food. This is only one factor within a wide range of various soil biological functions and yield-determining factors. Soil management practices that can improve crop production, such as no-till in water-limited environments, also work to improve soil properties like active carbon.
Q: Can I use active carbon to reduce fertilizer rates or modify plant population?
A: Active carbon has NOT been calibrated with fertilizer rate or plant population trials. So far, active carbon provides information on soil management . The relationships with crop productivity remain unclear. We must wait for more research to answer these yield-focused questions.
References
Weil, R.R., K.R. Islam, M.A. Stine, J.B. Gruver, and S.E. Samson-Liebig. 2003. Estimating active carbon for soil quality assessment: A simplified method for laboratory and field use. Am. J. Altern. Agric. 18(1):3–17.