Soil organic matter is a fundamental component of soil. It is comprised of living microorganisms, recently decomposed plant material, and stable humus organic compounds. Soil organic matter influences numerous biological, chemical, and physical properties of soil. It influences soil structure, water holding capacity, nutrient cycling, biological activity, and chemical fate and transport (e.g. pesticides). Soil organic matter is so important, you cannot really call something soil unless there is some organic matter present.
Soil organic matter is a fundamental component of soil. It is comprised of living microorganisms, recently decomposed plant material, and stable humus organic compounds. Soil organic matter influences numerous biological, chemical, and physical properties of soil. It influences soil structure, water holding capacity, nutrient cycling, biological activity, and chemical fate and transport (e.g. pesticides). Soil organic matter is so important, you cannot really call something soil unless there is some organic matter present.
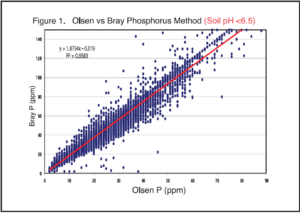
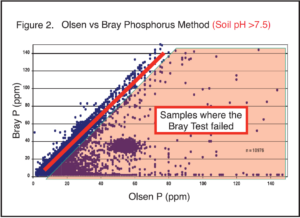
In the laboratory, soil organic matter can be measured via three different methods: Walkley-Black wet oxidation, estimation from organic carbon dry combustion, or loss-on-ignition (LOI) dry combustion. Each method has advantages and disadvantages. The LOI method is the routine method in commercial soil analysis.
Walkley-Black Wet Oxidation
The Walkley-Black wet oxidation method is the classic soil organic matter method developed in 1934. The method measures easily oxidizable carbon using sulfuric acid and potassium dichromate. The Walkley-Black method works very well on soils with low organic matter (<2.0%); however, the method is less suitable on soils with very high organic matter (>8.0%) as the dichromate reagent is consumed and may not oxidize all organic carbon. Soils with high chloride may interfere with the Walkley-Black method.
The Walkley-Black method has been phased out since the 1980s because the method requires hazardous chemicals and additional labor; therefore, the Walkley-Black method is more expensive than other methods. As the standard method, it is still required for some pesticide registration studies and regulatory soil characterization work.
Estimation from Organic Carbon Dry Combustion
Soil organic matter is a large complex organic compound containing hydrogen, oxygen, carbon, nitrogen, phosphorus, sulfur, and other elements. Soil organic matter contains about 58% organic carbon on average. We can estimate soil organic matter content from the amount of organic carbon measured in soil. Soil organic carbon is easily measured using a dry combustion carbon analyzer. The analyzer heats soil at high temperature to oxidize all carbon as carbon dioxide, which is then measured with an infrared detector. Since the method requires specialized instrumentation, it is more expensive than the LOI method.
The organic carbon dry combustion method is preferred in carbon sequestration research because organic carbon is measured directly. Estimating organic carbon from Walkley-Black or loss-on-ignition methods introduces unneeded calculation error.
For calcareous soils (pH > 7.3), inorganic carbon (carbonate) must also be analyzed. The dry combustion carbon analyzer measures total carbon, which combines inorganic and organic carbon. The inorganic carbon is measured separately, then subtracted from total carbon to calculate organic carbon.
Loss-on-Ignition Dry Combustion
The loss-on-ignition (LOI) dry combustion method is the routine soil organic matter method used in commercial soil analysis. The amount of soil organic matter is measured directly as the weight loss upon combustion at 360 deg C. The LOI method is simple, affordable, and safe. It also requires no hazardous chemicals. The method works well on soils with high organic matter content since there is no consumable reagent (like Walkley-Black method).
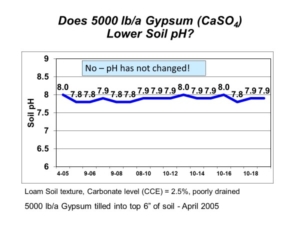
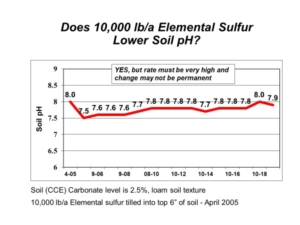
For soils containing hydrated salts (e.g. gypsum, CaSO4∙2H2O; Epsom salt, MgSO4∙7H2O), the LOI method may overestimate soil organic matter upon loss of water from hydrated salts. Soil is preheated at 105 deg C to remove structural water from clay minerals and hydrated salts, but some hydrated salts may retain water above the 105 deg C preheating process.
Soil organic matter affects various soil properties and processes. In return, various soil properties and soil formation factors affect soil organic matter.
Soil Texture
Soil clay particles protect soil organic matter from microbial decomposition (i.e. formation of protective clay-humus complexes). Soils with more clay generally have greater soil organic matter. In addition, soils with more clay also can store more plant available water, so plant biomass production and organic material addition to soil is greater.
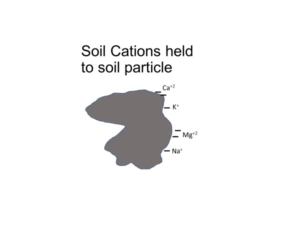
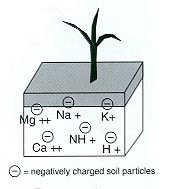
Cation Exchange Capacity
Cation exchange capacity is derived from negative-charged soil particles, like clay minerals and soil organic matter. Soils with more soil organic matter will have higher cation exchange capacity.
Bulk Density
Bulk density is the amount of soil mass per unit volume. Soils with high bulk density may be compacted, which can inhibit plant root growth and exploration of the entire soil volume. A compacted soil also has less open pore space for air and water storage and movement in soil. Soil organic matter has low particle density, and it helps alleviate soil compaction and high bulk density. Soils with low bulk density likely contain high soil organic matter.
Water Holding Capacity
The amount of soil organic matter in soil is closely associated with soil texture and bulk density. The association also extends to water holding capacity. Like clay particles, soil organic matter has a lot of surface area on which water films can adhere, thus increasing the water holding capacity. Soils with more soil organic matter will hold more plant available water than soils with low organic matter.
Soil pH
Soil microorganisms breakdown soil organic matter into its constituent nutrient components; this is called nutrient cycling. The type and species of soil microorganism present depend on soil pH. In neutral to alkaline soils, the soil microorganism community is comprised of diverse bacteria and fungi to decompose soil organic matter. In acidic soils, the soil microorganism community is mostly fungi, so decomposition processes occur slower. In acidic soils, soil organic matter accumulates faster, producing soils with high soil organic matter. Soils with very alkaline pH also have reduced soil microorganisms activity and decomposition rates.
Soil Microorganism Biomass
Soil organic matter is the primary carbon food source for soil microorganisms. To maintain a high amount of soil microorganism biomass and biological activity, a significant amount of soil organic matter is required. Soils with low organic matter generally have reduced biological activity.