Soybean cyst nematode (SCN) is a microscopic, parasitic worm that attacks the roots of susceptible soybean and dry edible bean, causing unseen or unexplained yield losses. Soybean and dry edible bean are naturally susceptible to SCN, but through plant breeding, most soybeans have some level of resistance, varying in level from good to poor. The most common source of resistance to SCN in soybean is PI88788, which is about 30 years old, and many soybean growing areas have SCN populations that are becoming resistant to this source. The Peking source is a very effective SCN resistance source but is only available in less than 5% of all soybean varieties.
Soybean cyst nematode cysts each harbor hundreds of eggs. Cysts and eggs of SCN can survive in the soil and remain viable for many years even without a soybean or dry bean host. Any activity that moves soil around will move SCN, meaning that areas with a history of soybean production likely have or will have this pest. Soybean cyst nematodes were first reported in Minnesota in 1978, South Dakota in 1995, North Dakota in 2003, and Manitoba in 2019.
During the growing season, the developing SCN cysts containing the eggs can be seen on susceptible plant roots, as seen in the picture below. To get an accurate assessment of the infestation level of the field, you need to collect soil samples and submit them to a laboratory to get a measure of the SCN egg count.
 Photo of soybean roots with SCN cysts. Photo courtesy of NDSU.
Photo of soybean roots with SCN cysts. Photo courtesy of NDSU.
Sampling strategies
If you have never tested for SCN before, you will want to sample fields intended for soybean or dry bean for the presence of SCN and gather a baseline SCN egg count. The best time to collect this sample is at the end of the growing season, right before harvest or just after (before any tillage). Sampling in the fall coincides with the highest egg levels in the soil and typically falls in the months of September and October. Collect 10-20 soil cores (6 to 8 inch soil depth) right in the soybean row from areas of the field that are likely to have SCN. Since SCN is a soil-borne pathogen, it moves wherever contaminated soil can enter the field. Therefore, the areas you will want to collect samples from are field entry points where soil can be transferred on equipment and tires, places where blown soil accumulates (e.g., fence lines), ditches and flooded areas, and locations in fields with consistently low soybean yields. Mix the soil cores together and take a subsample to fill a soil sample bag.
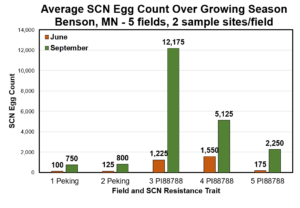
If you know you have SCN, you will want to sample soybean fields twice during the year: once in June to get an initial SCN egg count and then again in the fall to get a final SCN egg count. The early and late SCN samples allow you to measure if SCN populations are being effectively controlled (i.e., no increase in SCN egg count) or if the soybean variety SCN resistance source is failing (i.e., SCN egg count increases). Choose a single point in the soybean field and collect 8-10 soil cores (6 to 8 inch soil depth) taken within the soybean row at that spot. Mix the cores together and fill a regular paper soil sample bag. Mark that point with a flag and collect its GPS coordinates. Come back to that exact spot in the fall and collect a second sample. This will help you assess how your SCN management strategies, including the soybean variety SCN resistance source and soybean seed treatment, are working in the field.
Preparing and sending SCN samples to AGVISE Laboratories
You can submit SCN samples via paper form or online through AGVISOR. AGVISE provides special paper forms for SCN sampling and special stickers for online AGVISOR submission at no charge. The bright yellow forms and stickers help us sort samples and ensure samples submitted for SCN analysis are not dried and ground. All SCN samples analyzed by AGVISE Laboratories are analyzed at the Benson, MN laboratory. You can either send the SCN samples directly to the Benson Laboratory (see addresses below) or to the Northwood Laboratory, where they will be routed to Benson for analysis. AGVISE Laboratories reports SCN results in “eggs/100 cc” of soil and provides interpretation on our reports informed by university research.
Helpful links:
Soybean Cyst Nematode, ISU
Plant Disease Management: Soybean Cyst Nematode, NDSU
Soybean Cyst Nematode (SCN), UMN
Soybean Cyst Nematode in South Dakota: History, Biology, and Management, SDSU
The SCN Coalition
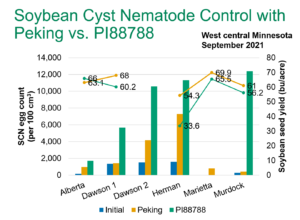 SCN egg count and soybean yield data from the 2021 AGVISE SCN resistance project. Bars of the graph represent SCN egg count, lines of the graph represent soybean yield. Click on the graph for a higher resolution version.
SCN egg count and soybean yield data from the 2021 AGVISE SCN resistance project. Bars of the graph represent SCN egg count, lines of the graph represent soybean yield. Click on the graph for a higher resolution version.