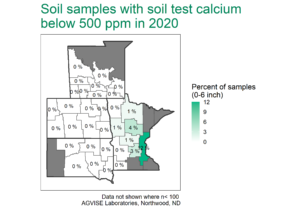
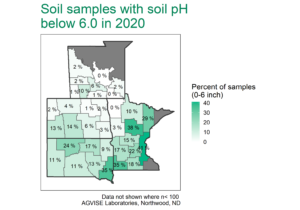
Uffda, that’s a lot of potash!
Question: Can you really change the %K base cation saturation ratio?
This winter, we have gotten more questions from farmers asking about the base cation saturation ratio (BSCR) concept. The farmers had attended a series of meetings where the speakers encouraged farmers increase the %K saturation in their soils and apply high potassium fertilizer rates to soils, even though there was little to no chance to get a crop yield response. In short, the BSCR concept revolves around reaching a certain percentage (%) of each base cation in your soil to obtain the “ideal” soil. If you do not have the right percentage of each cation, then you are instructed to apply large amounts of fertilizer to reach this “ideal” balance of each cation. Potassium is the most common nutrient where people fall into the BSCR trap, often suggesting that extra potassium will “fix” their soil.
Since the 1940s, university and industry researchers from around the world have thoroughly debunked the BCSR concept. But sometimes, you just need to show people how this works in the real world to get their attention. To help farmers see the silliness of the BSCR concept, we conducted a simple field project in 2015 to show just one flaw in the failed BSCR concept, focusing on its primary claim that you can actually change the %K saturation in soil. We identified three soils in Manitoba, Minnesota, and North Dakota with low initial %K saturation. The goal was to increase %K saturation into the 4 to 6% range, which is recommended by BCSR promoters. We applied a staggering rate of 1000 lb/acre K2O (1666 lb/ acre potassium chloride, KCl, 0-0-60). You are probably thinking that 1000 lb/acre K2O is a lot of potash! And so did we. We called the project the “uffda” project because my Norwegian grandfather, who farmed in southern Minnesota 60 years ago, would have looked at the high rate and said, “Uffda, that’s a lot of potash!”
But, we failed to achieve the “ideal” 4 to 6% K saturation (Table 1). As expected, soil test K (part per million, ppm) increased substantially, but the %K saturation did not reach the “ideal” soil range even with the enormous potassium fertilizer rate.
| Table 1. Soil test potassium and potassium base saturation after application of 1000 lb/acre K2O (1666 lb/acre potassium chloride, KCl, 0-0-60). | ||||
| Location | Soil test K, ppm | K saturation, % | ||
| initial | final | initial | final | |
| Northwood, ND | 156 | 430 | 0.6 | 1.6 |
| Benson, MN | 154 | 290 | 1.9 | 3.7 |
| Roseisle, MB | 50 | 330 | 0.4 | 2.8 |
Do you need more proof? Let’s see what the plants said. The soybean plant tissue K concentrations did not change either because the soil test K (ppm) was sufficient and above the 150 ppm critical level (Table 2). Simply put, the proven university-developed soil fertility guidelines would have told you that more potassium was not needed. I know a few farmers who were convinced to follow the BCSR concept, which only wasted their valuable dollars and time on the failed idea.
| Table 2. Soybean leaf potassium concentration did not increase with additional potassium fertilizer applied on soils with soil test potassium above the critical level (150 ppm). | ||
| Fertilizer K Rate | Soybean leaf K, % | |
| lb/acre K2O | Northwood, ND
STK 150 ppm |
Benson, MN
STK 190 ppm |
| 0 | 3.2 | 1.9 |
| 20 | 2.6 | 1.8 |
| 100 | 2.7 | 1.8 |
| 200 | 3.0 | 1.8 |
| 1000 | 2.6 | 1.9 |
| Soybean leaf K sufficiency level = 1.7 %K | ||
All things considered, there are still good reasons to apply potassium fertilizer (moderate rates) to achieve profitable yield responses. Either way, the base cation saturation ratio (BCSR) concept is a really bad way to justify potassium fertilizer use, and it usually leads to very high fertilizer rates and costs. Here are some good reasons to include potassium in your soil fertility program:
- Soil test K below 150 ppm (grid or zone soil test)
- Soil test K below 200 ppm (composite soil test, high soil variability)
- History of low plant tissue K when no potassium fertilizer is applied; potential compaction
- Replicated strip trials showing profitable crop yield response to moderate potassium fertilizer rates
- Chloride required for small grains; potassium chloride (0-0-60-50Cl) is the most common chloride source