Soil Science Review: Cation Exchange Capacity
Each year, AGVISE Laboratories delivers thousands of soil characterization reports with something printed on them called, “Cation Exchange Capacity (CEC).” Unless you have some background in soil science or surface chemistry, the number might be a mystery to you. Cation exchange capacity is the amount of positive-charged cations (e.g. ammonium, calcium, hydrogen, magnesium, potassium, sodium) held on negative-charged soil particles, like clay and organic matter.
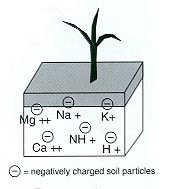 Let’s start with the basics. Elements and compounds in soil usually exist as ions, which have either positive or negative charge. The positive ions are called cations. Some common positive-charged cations are calcium, magnesium, potassium, and sodium.
Let’s start with the basics. Elements and compounds in soil usually exist as ions, which have either positive or negative charge. The positive ions are called cations. Some common positive-charged cations are calcium, magnesium, potassium, and sodium.
Soil particles have negative charge on their surfaces and edges. Since soil particles have negative charge and the cations have positive charge, the two are attracted together like magnets. If the positive-charged cations are held on the negative-charged soil particles, then the ion cannot leach through the soil profile with soil water. Therefore, the amount of positive-charged cations that are held on negative-charged soil particles is the cation exchange capacity (CEC). The CEC reporting units are centimole of charge per 1 kilogram soil (cmolc/kg) or milliequivalent per 100 gram soil (meq/100 g). The units are numerically equivalent, so a soil with CEC 20 cmolc/kg is equal to 20 meq/100 g.
Soils with high CEC are generally more fertile and can provide plants with more nutrients and water. A soil with high CEC (>25 cmolc/kg) can hold many cation nutrients and likely contains a high amount of clay and/or organic matter. A soil with low CEC (<5 cmolc/kg) cannot hold many cation nutrients, and it is likely sandy with little organic matter.
The CEC measurement can also provide information about the fate and transport of other charged compounds in the soil solution, like pesticides. Pesticides with positive charge are bound more tightly to soil particles if CEC is high. Sandy soils with low CEC often cannot hold onto positive-charged pesticides, meaning that pesticide may be prone to leaching.
There are different laboratory methods to measure CEC of soil. The most common method in commercial soil testing is the summation method, where all extractable cations on soil particles are summed together. The cations are extracted with ammonium acetate, then analyzed with atomic absorption spectroscopy (AAS) or inductively coupled plasma atomic emission spectroscopy (ICP-AES). The extracted cations are then added together to calculate CEC. The routine method works well on most soils; however, it does not do work well on soils with salinity or calcium carbonate (pH > 7.3). The ammonium acetate extraction also dissolves cations contained in soluble salts and calcium carbonate minerals. These cations are not held on cation exchange sites (i.e. cation exchange capacity), but they are still included in the CEC measurement, creating an inflated and inaccurate CEC result.
To obtain accurate CEC results on soils with salinity or calcium carbonate, the saturation-displacement CEC method is appropriate. The method first saturates all cation exchange sites with one cation (either ammonium or sodium) and washes away all other cations. The second step displaces the target cation to obtain the accurate CEC measurement. The saturation-displacement CEC method involves more work and cost than the routine summation method. You should talk with a soil scientist to help you decide which laboratory method is required to obtain an accurate CEC result.
