High soil pH and calcium carbonate inflate base cation saturation and cation exchange capacity (CEC)
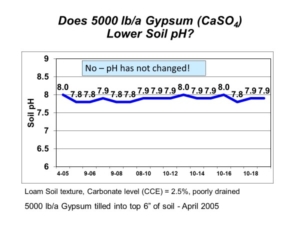
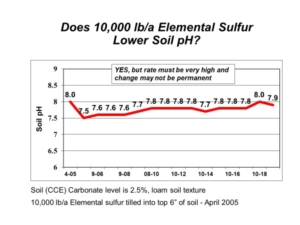
Soil pH is a soil chemical property that measures soil acidity or alkalinity, and it affects many soil chemical and biological activities. Soils of the northern Great Plains and Canadian Prairies often have high soil pH (>7.3) and contain calcium carbonate (free lime) at or near the soil surface. It is the calcium carbonate in soil that maintains high soil pH and keeps it buffered around pH 8.0. The calcium carbonate originates from soil formation processes since the latest glacial period.
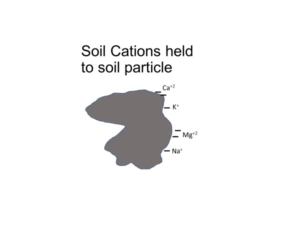
Soils with high pH and calcium carbonate create analytical challenges in determining cation exchange capacity (CEC) and subsequent base cation saturation ratio (BCSR) calculations. Cation exchange capacity is the amount of positive-charged cations (e.g. ammonium, calcium, hydrogen, magnesium, potassium, sodium) held on negative-charged soil particles, like clay and organic matter. Fine-textured soils (high clay content) and organic soils (high organic matter content) have high CEC, while coarse-textured soils (low clay content) have low CEC. The BSCR is the relative proportion of base cations in soil.
The routine laboratory method to determine CEC is the summation method, where all extractable cations on soil particles are added together. The assumption is that all positive-charged cations extracted are held on negative-charged exchange sites on soil particles. The assumption falls apart in soils with pH > 7.3 because the soil test method also extracts calcium from the naturally occurring soil mineral calcium carbonate, which is not held on cation exchange sites. The resulting amount of extractable calcium is inflated. The summation procedure still sums together the inflated calcium result, producing an inaccurate and inflated CEC result. Any subsequent base cation saturation calculations are similarly flawed.
To illustrate the inflated CEC problem, AGVISE Laboratories conducted a laboratory experiment. The experiment showed that more calcium carbonate in soil increased the amount of extractable calcium (Table 1). Furthermore, the inflated amount of extracted calcium also inflated the CEC because all the extractable cations are summed together. The correct CEC is 24 cmolc/kg, yet the inflated CEC could be 150 to 200% higher with increasing calcium carbonate content. Adding calcium carbonate to soil did not increase the inherent CEC sources, i.e. clay and organic matter, yet the laboratory CEC result increased. In reality, the ability to hold more cations did not change. This highlights the analytical challenge in determining CEC via summation method on soils with high pH and calcium carbonate.
| Table 1. Effect of calcium carbonate addition on extractable cations and cation exchange capacity. | |||||||
| Calcium carbonate equivalent (CCE) | pH | EC | Ca | Mg | K | Na | CEC |
| % | dS/m | —————– ppm —————– | cmolc/kg | ||||
| 0 | 7.5 | 0.43 | 3350 | 730 | 220 | 45 | 24 |
| 1 | 7.6 | 0.51 | 6150 | 730 | 220 | 50 | 37 |
| 5 | 7.7 | 0.52 | 7480 | 720 | 215 | 45 | 44 |
| 10 | 7.8 | 0.50 | 7240 | 650 | 180 | 40 | 42 |
| Abbrev.: pH (1:1); EC, electrical conductivity (1:1); Ca, calcium; Mg, magnesium; K, potassium; Na, sodium; CEC, cation exchange capacity via summation of cations. | |||||||
The subsequent base cation saturation calculations were also affected, showing much lower percent potassium saturation as calcium carbonate content increased (Table 2). This presents a major challenge in using the base cation saturation ratio (BCSR) concept to guide soil fertility and plant nutrition on soils with high pH and calcium carbonate. Without an accurate analytical method, the BCSR concept loses its grounding and any practical application.
| Table 2. Effect of calcium carbonate addition on base cation saturation ratio and cation exchange capacity. | |||||
| Calcium carbonate equivalent (CCE) | Ca sat. | Mg sat. | K sat. | Na sat. | CEC |
| % | ————————– % ————————– | cmolc/kg | |||
| 0 | 71 | 26 | 2.4 | 0.8 | 24 |
| 1 | 82 | 16 | 1.5 | 0.6 | 37 |
| 5 | 85 | 14 | 1.2 | 0.4 | 44 |
| 10 | 86 | 13 | 1.1 | 0.4 | 42 |
| Abbrev.: Ca, calcium; Mg, magnesium; K, potassium; Na, sodium; CEC, cation exchange capacity via summation of cations. | |||||
In general, the routine CEC method via summation of cations is quite accurate on soils with pH less than 7.3 and no calcium carbonate. However, the routine method has clear challenges on soils with pH greater than 7.3. The resulting CEC and subsequent base cation saturation calculations are inflated and/or flawed. This is why the BCSR concept is extremely misleading on soils with high pH.