Fall-applied Nitrogen Fertilizer: A Couple Simple Rules
This article is shared annually to help answer frequently asked questions about nitrogen fertilizer applications and nitrogen losses in the fall.
October is here, and many people are preparing for fall nitrogen fertilizer applications. Before you hit the field, we want to share these important reminders about fall nitrogen application timing and placement to help you reduce potential soil nitrogen losses through fall and winter.
It is important to wait until soil temperatures reach 50 °F (10 °C) before applying fall nitrogen to reduce the risk of soil nitrogen loss. Once nitrogen fertilizer is applied, soil microbes begin converting ammonium-nitrogen (NH4+) to nitrate-nitrogen (NO3-), a process called nitrification. In the nitrate form, nitrogen is vulnerable to loss through nitrate leaching or denitrification. Soil temperatures cooler than 50 °F help slow microbial activity and keep nitrogen in the safer ammonium-nitrogen form longer. This applies to any ammoniacal nitrogen fertilizer source, which includes anhydrous ammonia, urea, UAN, and ammonium sulfate.
Quick rules for fall-applied nitrogen timing
- Wait until after October 1 because cool soil temperatures are more consistent and reliable after this date. After October 1, measure soil temperature in the early morning (6 a.m. to 8 a.m.) at the 4-inch soil depth.
- When the 4-inch soil temperature has reached 50 °F (10 °C), it is relatively safe to start applying anhydrous ammonia.
- Wait one week after the anhydrous ammonia-safe date to apply banded urea.
- Wait two weeks after the anhydrous ammonia-safe date to apply broadcast urea.
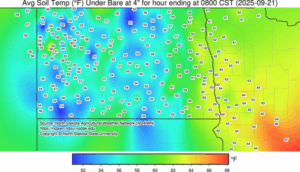
Soil temperature map from the North Dakota Agricultural Weather Network (NDAWN) from 21 September 2025. You can find an updated daily soil temperature map at the NDAWN website.
It is a good idea to keep a soil thermometer with you to measure the current soil temperature in the field. In addition to NDAWN, a number of regional climate mesonets have online tools to search for local and regional soil temperatures.
- Alberta Climate Information Service
- Manitoba Agriculture – Soil Temperature Trends
- Minnesota Department of Agriculture – Soil Temperature Map
- Montana Climate Office Mesonet
- NWS North Central Region – Soil Temperature Map
- North Dakota Agricultural Weather Network (NDAWN) – Soil Temperature Map
- South Dakota State University Mesonet
The 50 °F soil temperature rule of thumb is particularly important for soils prone to nitrogen loss: well-drained, coarse-textured soils are prone to nitrate leaching and poorly-drained, fine-textured soils are prone to denitrification. If such soils receive excess precipitation or become saturated (waterlogged) through fall or spring, soil nitrate can be lost through leaching or denitrification. In general, it might be better to apply nitrogen fertilizer on such soils in spring. But, if you must apply nitrogen fertilizer in the fall, make sure you wait until soil temperatures are cold enough to keep it in the ammonium-nitrogen form for a longer period of time to reduce potential soil nitrogen losses.
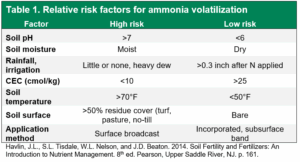
For fall-applied nitrogen, subsurface banding or incorporation is also important to reduce ammonia volatilization, another potential nitrogen loss mechanism. Fall precipitation (rain or snow) is often too sporadic and unreliable to be considered an effective incorporation “strategy” for broadcast applications. Fall-applied urea should be banded below the soil surface (3 inches or deeper) or incorporated with tillage (at least 3-4 inches) to ensure complete coverage.
Shallow fertilizer bands or shallow incorporation with vertical tillage does not provide adequate soil coverage to prevent ammonia volatilization. If soils are very dry, successful incorporation may not be possible because tillage can produce large, uneven clods that leave nitrogen fertilizer exposed to the atmosphere and vulnerable to ammonia volatilization. Although dry soil poses a lower risk of ammonia volatilization than moist soil, soil moisture is not the only factor that contributes to ammonia volatilization risk (Table 1).
Fall-applied anhydrous ammonia should be banded 5 to 6 inches deep. Ensure that anhydrous ammonia trenches are sealing properly to prevent gaseous ammonia losses from the trench. In addition, the nitrification inhibitor nitrapyrin (brand name N-Serve) can be added to anhydrous ammonia to slow nitrification, offering additional insurance to keep nitrogen in the safer ammonium-nitrogen form for longer. Nitrapyrin is also available in formulations for dry and liquid nitrogen products. Please note that nitrapyrin degrades faster and loses its effectiveness at warmer soil temperatures, so it is no substitute for cooler soil temperatures (<50 °F).
Fall-applied nitrogen is a convenient way to allocate time and labor resources, leaving one less thing to do in the spring. But, you must be smart and consider fertilizer source, timing, and placement options to make sure that the nitrogen applied in the fall will still be there next spring. With fertilizer prices still remaining high, now is not the time to risk soil and fertilizer nitrogen loss.